On March 8th the world celebrated International Women’s Day. On March 10th the breast cancer community celebrated the FDA’s final rule standardizing breast density inform requirements across the country. This is a long-awaited step forward in breast cancer early detection. These related events speak to a historic moment for women’s health.
We are on the precipice of a time when women are empowered with information that can help find breast cancer earlier. Breast density, an independent risk-factor that increases a woman’s likelihood of developing breast cancer and obscures clear images on a mammogram, affects almost 50% of women. Women with dense tissue need screenings in addition to mammograms. Until March 10th, only 38 states and the District of Columbia had existing laws mandating that information about a patient’s breast density be included in mammogram reports. Additionally, the wording and requirements of these laws varied across the country. This created confusion and inconsistencies for women.
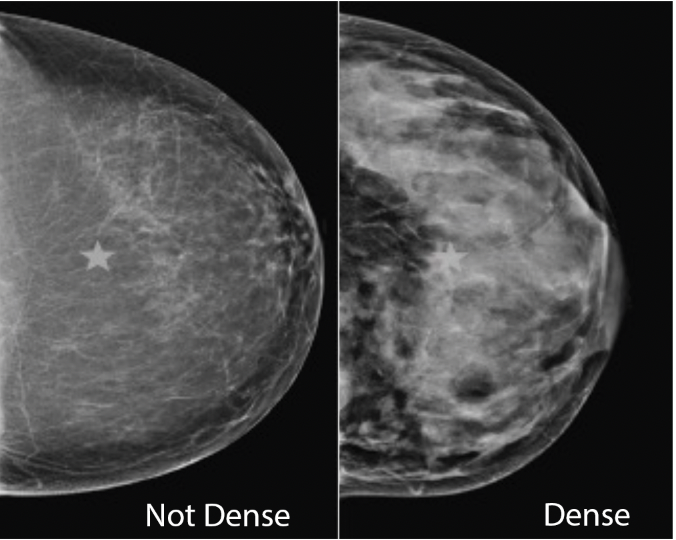
On March 10, 2023, the FDA issued a final rule which requires that all mammogram reports inform women of their breast density, indicate that breast density is a risk factor for breast cancer, and advise that patients with dense tissue speak with their providers about screenings beyond mammograms. This is cause for celebration, but it must be put into context.
We still have a long way to go before all women with dense breast tissue can access the comprehensive screenings they desperately need and deserve. Women who have dense breasts need lifesaving screenings in addition to mammograms. These often include ultrasounds and/or MRI’s. In the general population, mammograms are about 85% effective at detecting breast cancer. For women with dense tissue, mammograms are only 56% effective. However, when mammograms are combined with ultrasounds in women with dense breast tissue, cancers are found 88% of the time. This is a major, lifesaving difference.
Now that there is a universal breast density inform law, all women will know if they have dense breasts, and that dense tissue is a risk factor that requires extra screening. But access to essential screenings continues to be a barrier for many women. Nothing in the federal density-inform rule requires insurance coverage for additional lifesaving screenings. This disparity creates a landscape in the United States where women who can pay out of pocket get their lifesaving tests but women who cannot, fall through the cracks.
While we applaud the progress that Congress and the FDA have made towards a world where breast density is understood and addressed, we have a long way to go. To date, sixteen states and the District of Columbia1 have laws that require insurance to cover screenings beyond mammograms for higher risk women, including those with dense tissue. These states chose to address the medical, financial, and social justice benefits of offering all higher-risk women access to lifesaving exams. From a cost perspective alone, 12 months after diagnosis, a stage I diagnosis costs about $26,424, compared with $116,087 for stage IV.2 By year 4 post diagnosis, Stage 1 costs, on average, $85,000 but Stage 4 costs approximately $550,000.3 The survival rates are comparably bleak for later stage disease. Five years post diagnosis the survival rate for stage 0/I is about 99% compared with about 30% for Stage IV.4 It is important to note that these mortality rates and costs span up to five years, however many cancer survivors incur staggering life-long costs due to ongoing doctors’ appointments, imaging, and other tests. It is, therefore, advantageous morally, medically, and financially to cover screenings beyond mammograms for women with dense breasts.
This March, during Women’s History Month, let’s use the FDA final rule to spur us towards action. Now that all women will be educated about dense tissue, it is time to galvanize our communities to ensure that this knowledge leads to action. The best way to do that is to support the Find It Early Act, which would create a federal law requiring insurance coverage for screenings for higher-risk women. We need to follow the lead of the sixteen states and DC to ensure that women, regardless of their socio-economic status, can access the lifesaving, essential breast screenings they need and deserve.
Visit these links to learn more about breast density
References:
1. https://densebreast-info.org/legislative-information/state-legislation-map/
2. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-022-08457-6
3. Id.
4. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html

