Scorecard™
Volpara Scorecard™ gives your breast care team the insights they need to find cancer earlier. Improve clinical decision-making with key breast density and risk insights in one view.
Request a demo
The Volpara Health approach to trusted, effective AI
Explore Volpara’s AI eBook and learn how to evaluate AI innovations for your practice.
New FDA National Density Reporting Standard
The Food & Drug Administration has updated the Mammography Quality Standards Act (MQSA) to make patient breast density notification a federal requirement.
20 m+
Women across 40 countries have had their breast composition assessed by Volpara TruDensity
The only
Volumetric breast density assessment validated for use with Tyrer-Cuzick 8
Decision Support
Triage women for additional breast screening
Science-based data and alerts when patients meet high-risk thresholds for additional detection or preventative care.

Risk Insight 1
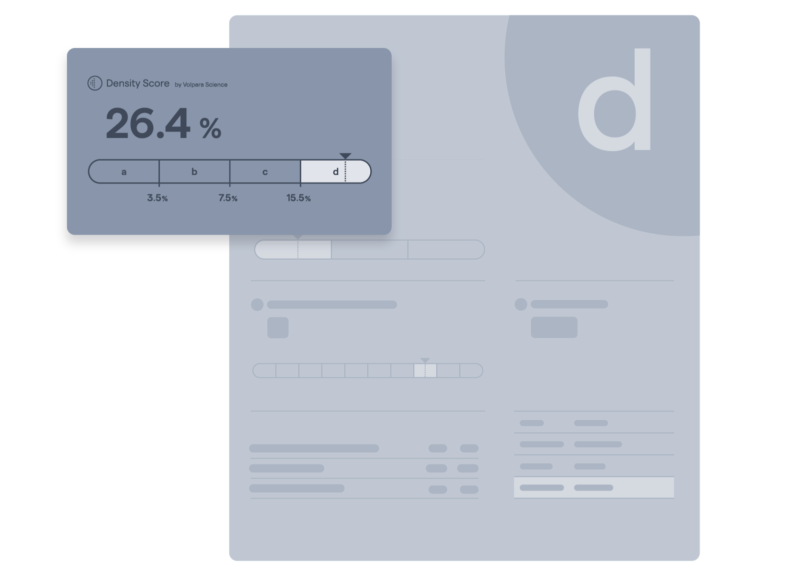
Automatic breast composition assessment
Volumetric density measurements validated for Tyrer-Cuzick 8, offering objective, precise, and consistent breast cancer risk assessment.
Risk Insight 2
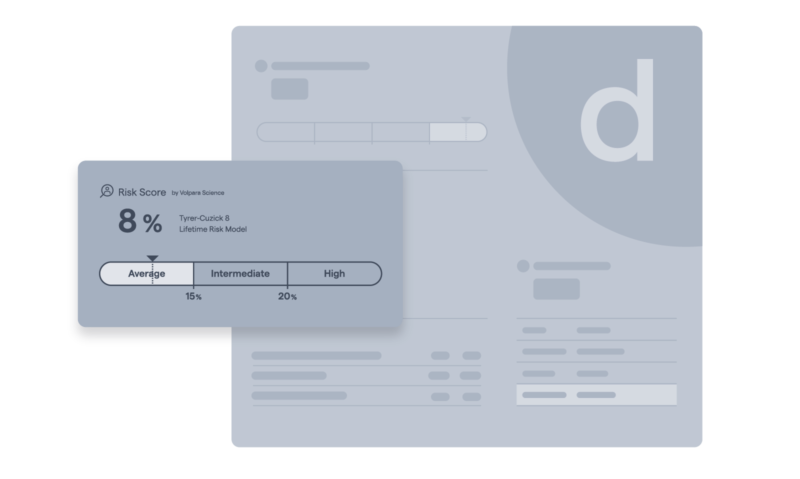
Lifetime breast cancer risk calculator
Integrated Tyrer-Cuzick 8 risk modeling calculates lifetime risk of developing breast cancer.
*requires additional software purchase, not available in all countries
Risk Insight 3
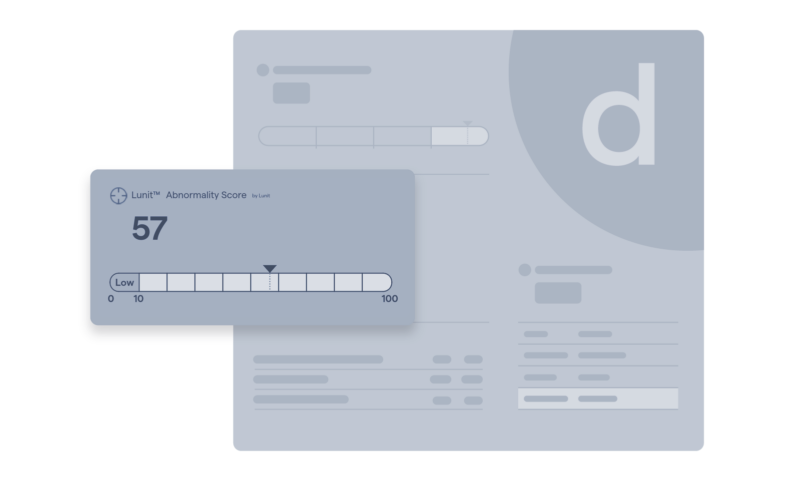
Suspicious findings
AI and machine learning categorize mammograms by likelihood of cancer.
*requires additional software purchase, not available in all countries
Product info pack
Detailed product information and data sheet.
Interested? Request a demo or consultation.
Explore
You might be interested in...

eBook: AI that works in breast imaging

FDA national breast density notification requirement
Blog

30 Mar 2023
The Federal Breast Density Inform Rule: Huge Leap Forward, But More To Do
News

10 Mar 2023
FDA Breast Density Reporting Rule is a Critical Step for Women says Volpara Health, the Leader in AI-assisted Breast Density Measurement


