Volpara software is used to assess the breast density of more than 6 million US patients annually
Lynnwood, WA, March 10, 2023: Volpara Health Technologies (“Volpara,” “the Group,” or “the Company”; ASX:VHT), a global leader in software for the early detection of breast cancer, today announced that a new US federal regulation was finalized by the US Food and Drug Administration (FDA) requiring mammography facilities across the country to inform patients whether their breasts are composed of dense tissue. The regulation standardizes language and expands the number of states with density disclosure laws nationwide.
National Notification Ruling
Within the next 18 months—by 10 September 2024—all mammography patient reports and summaries must include the following language about breast density to inform the patient of their density status as either non-dense or dense:
- Non-dense breast notification states: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is not dense. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.
- Dense breast notification states: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is dense. In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”
The ruling also specifies the language about breast density in reports and summaries for healthcare providers is to match the BI-RADS® 5th Edition density categories.
“The FDA breast density notification language is a key step in equitably empowering all women in the United States to understand their breast density so they can take informed, actionable steps to monitor their own breast health,” said Teri Thomas, CEO of Volpara Health.
Opportunities for Further Accuracy & Empowerment
Nearly 40 million mammograms are performed each year in the US of which Volpara’s software is used to assess the breast density of more than 6 million annually.
The FDA ruling acknowledges advancements in density classification devices to help mitigate variability in assessment.
Volpara’s volumetric breast density assessment software to support physicians has long played an increasingly important role in making accurate, objective assessments of breast density possible. Through the use of AI (artificial intelligence), Volpara provides a comprehensive and precise measurement of breast density that helps ensure breast cancer risk is more accurately assessed. The Volpara® TruDensity™ physics-based AI algorithm is cleared by the FDA, Health Canada, and the TGA (Australia), is CE-marked, and has been validated in more than 400 articles and research abstracts.
“We’ve been working with leading clinicians and researchers around the world for more than a decade to make critical information about women’s breast composition and its link to breast cancer more readily available,” said Thomas. “The FDA regulation validates our focus, increases the industry’s attention on breast density, and propels society forward to improve both the patient and provider experience and understanding.” 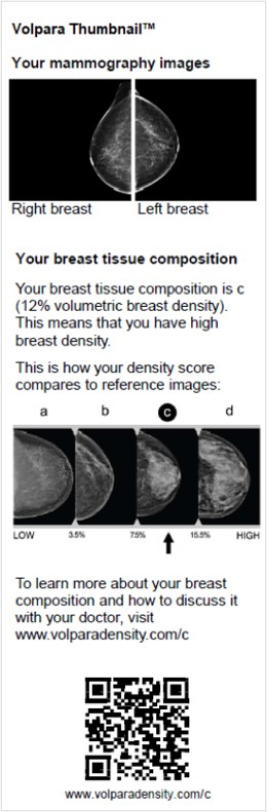
Volpara’s Thumbnail™ module enhances patient mammography results letters with two mammogram images of the patient’s breasts and explains the meaning of breast density in simple-to-understand terms. These additions beyond notification advance patient communication to help providers go the extra mile to help patients. Volpara users also have access to tools which help them further educate patients, referring physicians and their community. Informative brochures, posters, staff scripts and other educational aids may be used as is or be customized by the provider. Of note, Volpara has created an educational website about breast density that includes a gamification feature to allow visualization of how cancer can be obscured in dense breasts.
This tool can be viewed at www.volparadensity.com.
About Breast Density
Dense breast tissue is common but has been linked to an increased risk for breast cancer and can also dramatically impact early detection. In the United States, nearly half of all women over 40 have dense breasts. As density increases, the accuracy of mammography decreases. According to a study published in Radiology, mammography misses almost half of breast cancers in women with the densest breasts. Because dense breast tissue and cancer appear white on a mammogram, tumors are often camouflaged on a mammogram. Studies confirm that early detection improves when women with very dense breasts receive an ultrasound or MRI exam in addition to mammography as part of their regular screening schedule.
For further information, please contact:
MaryAnne Molter
Senior Marketing Manager
Volpara Health
MaryAnne.Molter@VolparaHealth.com
414-339-9900
About Volpara Health Technologies Limited (ASX:VHT)
Volpara Health Technologies makes software to save families from cancer. Healthcare providers use Volpara to better understand cancer risk, empower patients in personal care decisions, and guide recommendations about additional imaging, genetic testing, and other interventions. Our AI-powered image analysis enables radiologists to quantify breast tissue with precision and helps technologists produce mammograms with optimal image quality, positioning, compression, and dose. In an industry facing increasing staffing shortages, our software streamlines operations and provides key performance insights that support continuous quality improvement.
Volpara is the preferred partner of leading healthcare institutions around the world. Our software is used in over 2,000 facilities by more than 5,000 technologists, impacting nearly 16 million patients globally. It helps providers conduct more than three million cancer risk assessments each year and can be deployed stand-alone or fully integrated with electronic health record systems, mammography reporting systems, imaging hardware, and genetic laboratories. Volpara holds the most rigorous security certifications and numerous patents and regulatory registrations, including FDA clearance and CE marking. Since listing on the ASX in April 2016, the Company has raised A$132 million. With an office in Seattle, Volpara is based in Wellington, New Zealand.
For more information, visit www.volparahealth.com.
